1. INTRODUCTION
Hydrostatic pressure regulation within organs is a critical physiological parameter, influencing both normal function and pathological progression. Abnormal pressure dynamics are closely linked to the onset and progression of various diseases. For instance, elevated intracranial pressure is a hallmark of hydrocephalus and traumatic brain injury, while increased intraocular pressure is the primary risk factor for glaucoma [1,2]. Similarly, compartment syndrome arises from pressure accumulation in muscle compartments, leading to ischemia and potential tissue damage if not detected early. These examples underscore the need for accurate, continuous pressure monitoring for both diagnostic and therapeutic purposes.
Beyond neurological and Musculo-skeletal disorders, pressure monitoring has gained increasing importance in oncology. Interstitial fluid pressure (IFP) in solid tumors is frequently elevated due to abnormal vascular structures and impaired lymphatic drainage. High IFP acts as a barrier to the effective delivery of chemotherapeutic agents and contributes to a hostile tumor microenvironment that fosters invasion, metastasis, and resistance to therapy [2]. Real-time monitoring of IFP could enable dynamic adjustment of treatment strategies and early prediction of therapeutic outcomes, offering significant clinical value in cancer management.
Despite its importance, continuous hydrostatic and interstitial pressure monitoring remains technically challenging. Conventional pressure sensors, such as catheter-based devices or strain gauges, are typically rigid, invasive, and poorly suited for implantation in soft or confined biological environments. These limitations restrict their long-term use and compromise patient safety. MEMS-based capacitive sensors provide an alternative due to their small form factor and potential for integration; however, they are often difficult to fabricate at high sensitivity levels, especially when large capacitance is required. Moreover, their small sensing areas render them susceptible to instability and noise from local mechanical stress.
To overcome these challenges, there is a growing interest in developing soft, conformal pressure sensors using biocompatible and flexible materials. Among these, polydimethylsiloxane (PDMS) is widely used due to its mechanical compliance, chemical stability, and ease of processing. Enhancing the dielectric properties of PDMS through the incorporation of high-permittivity materials such as barium titanate (BaTiO3) nanoparticles offers a promising route to increasing sensor sensitivity. Furthermore, introducing porosity into the PDMS matrix using sacrificial templates like sodium chloride can improve compressibility, allowing for greater deformation under physiological pressure loads.
In this study, we present a porous PDMS-based flexible capacitive pressure sensor integrated with a Guyton chamber—a design that mimics a physiological fluid environment to accurately transmit pressure. The porous architecture enhances sensor responsiveness by increasing compliance, while the embedded BaTiO3 (BTO) nanoparticles significantly improve the dielectric constant, resulting in higher capacitance shifts under pressure. The sensor is designed for minimally invasive deployment and is capable of detecting hydrostatic pressure changes with high linearity and repeatability. We demonstrate the effectiveness of this sensor for potential use in biomedical contexts, including intra-organ pressure measurement and tumor IFP monitoring, providing a foundation for future integration into clinical diagnostic and therapeutic systems.
2. METHODS
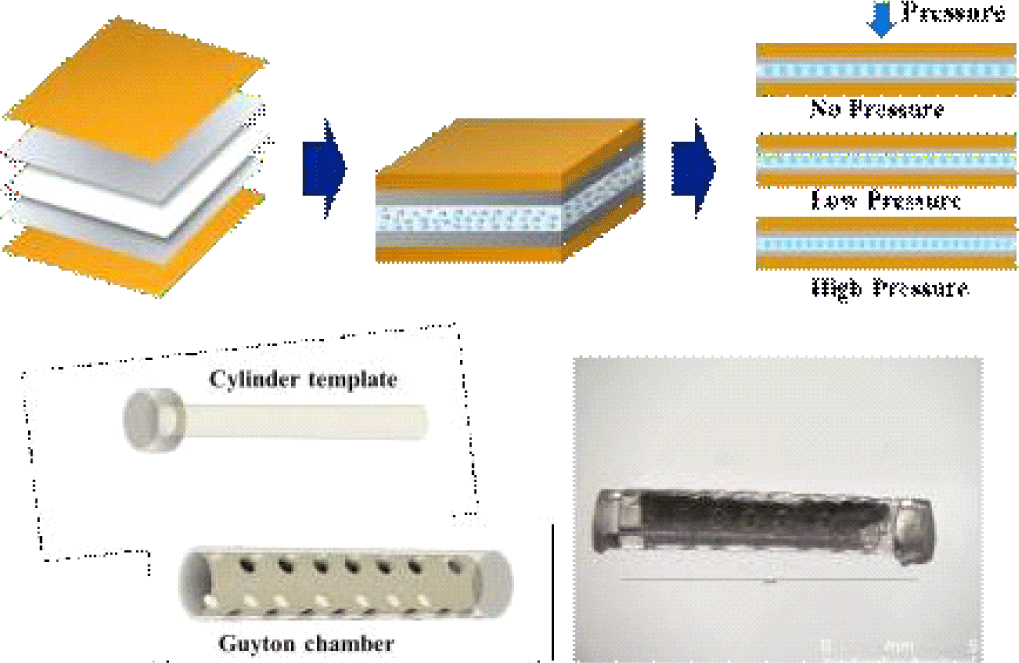
The porous BTO-PDMS was chosen as the dielectric materials for the capacitive pressure sensor; the cubic perovskite BTO is a popular dielectric material due to its large dielectric constant and has been widely used to increase the dielectric constant of polymeric materials [4,5]. Introducing the porosity or geometric features within the polymeric elastomer is another strategy to increase the mechanical deformation of the polymer via collapsing of the pores or structures [6-8]. In hydrostatic measurements, the pores can play an additional role by trapping and expelling water from the material. Since, physiological fluid is a lossy dielectric with a high dielectric constant over 80, the trapping and expelling of the water droplets within the pores can increase the pressure sensitivity. In addition, the flexible nature of the capacitive sensors allows the sensor to be rolled into a cylindrical Guyton chamber, which insulates mechanical pressures from movements or organ movements, for more accurate hydrostatic pressure measurements.
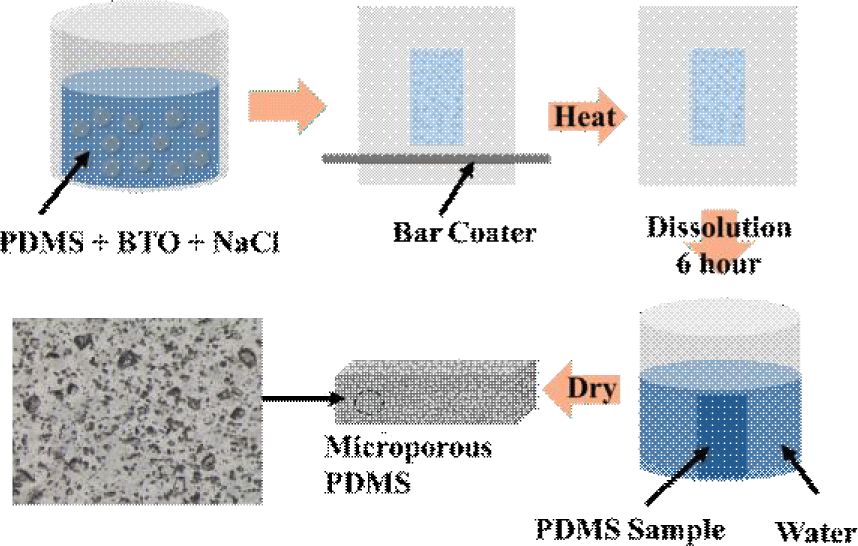
The fabrication steps for the porous composite of PDMS and BTO nanoparticles (U.S. materials) are shown in Fig. 2. Sodium chloride (NaCl) was incorporated as a sacrificial template to induce porosity. First, a uniform mixture of PDMS prepolymer, curing agent, BTO nanoparticles, and NaCl particles (sieved to collect particles with diameter<50 μm) was prepared and poured onto a polyethylene terephthalate (PET) substrate. A thin film (of 50 μm) was prepared by using a bar coater and cured in a convection oven. The composite was thermally cured and then immersed in deionized water to dissolve the NaCl for 6 hours, leaving a porous structure. BTO was used to enhance the dielectric constant, thereby improving sensor sensitivity, while the porous architecture enhanced mechanical compliance under hydrostatic pressure.
The pressure sensing elements were fabricated by coating the porous BTO-PDMS. Two electrode materials were used; silver and PEDOT:PSS. a conductive polymer blend of poly (3,4-ethylenedioxythiophene) and poly (styrenesulfonate). For the PEDOT:PSS coating, first, a conductive solution (Solution A) was prepared by mixing PEDOT:PSS, Triton X-100, and ethylene glycol (EG) in a volume ratio of 83:10:7. This solution was then blended with PDMS at a ratio of 2:1 to form the coating mixtures. Prior to coating, the BTO-PDMS substrates underwent oxygen plasma treatment to enhance surface adhesion. The prepared mixture was poured onto the treated substrate, then tilted in all directions to achieve a uniform thin coating. The coated samples were left at room temperature for one hour, followed by baking in an oven at 100°C for another hour to complete the curing process. Lastly, the sensor was encapsulated in polyimide films for mechanical stability and electrical insulation (Fig. 3(a)). We note that spin-coating was avoided due to poor adhesion and non-uniform coverage on the porous surface; instead, a tilt-and-cure method was adopted to achieve conformal electrode formation with improved contact stability. In addition, we note that the thickness of the PEDOT:PSS was not very critical, although it can affect the RC time constant, for the low-frequency pressure measurement.
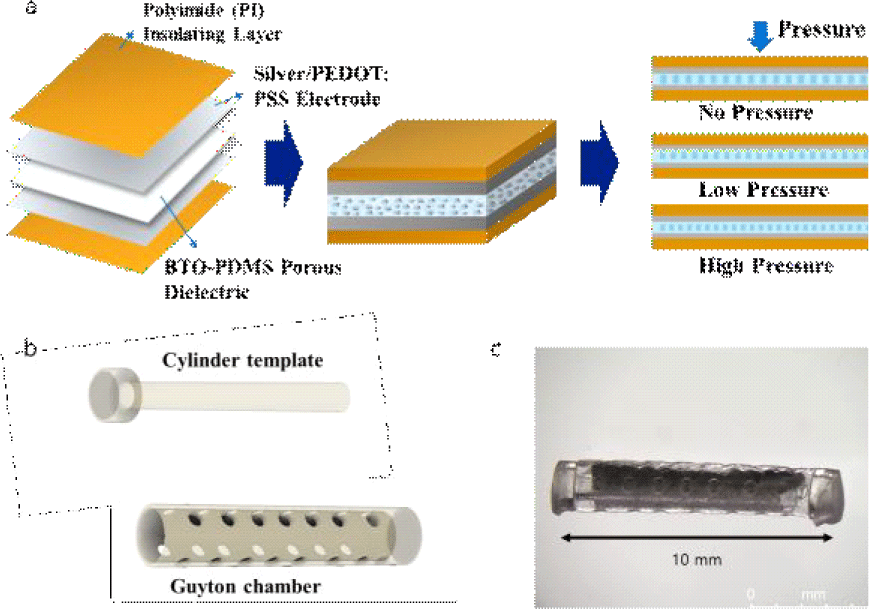
Subsequently, the sensor was wrapped around the 3D printed cylinder template and was assembled with a Guyton chamber. Both cylinder template and Guyton chamber were printed using a SLA-type 3D printer (Form4, Formlabs, USA) as shown in Fig. 3(b). The optical photograph of the assembled sensor is shown in Fig. 3(c). The overall sensor dimension was 2 mm in diameter and 12 mm in length. The cylindrical form factor is suitable for implantation via biopsy needle.
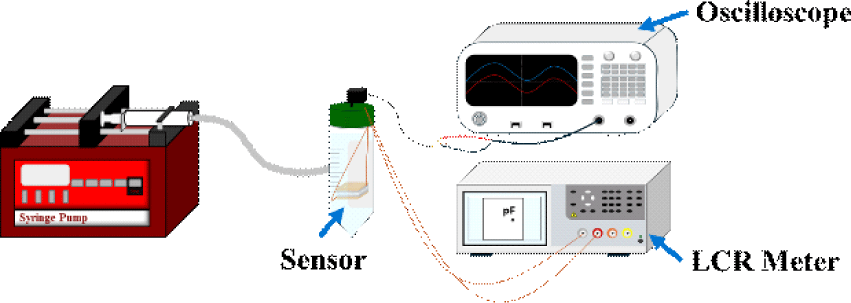
Capacitance was measured using an LCR meter (100 kHz), with the sensor immersed in water to simulate hydrostatic pressure conditions. A syringe pump was used to modulate the water pressure in a controlled manner. The capacitance response was recorded during both pressure application (compression) and release (retraction), and a reference pressure sensor (PSS-1V, Autonics, South Korea) was used to ensure accurate pressure readings as illustrated in Fig. 4.
3. RESULTS AND DISCUSSION
Optical microscopy images illustrate the morphological variations in PDMS samples influenced by both NaCl-induced porosity and the inclusion of BTO nanoparticles. In the Low-salt, non-BTO sample (Fig. 5(a)), the PDMS matrix exhibits clear, well-defined pores, formed via the dissolution of NaCl microparticles initially mixed into the matrix. Food coloring dye, co-loaded with the salt, visibly marks the original salt locations, assisting in identifying regions of successful pore formation. Despite some residual salt presence, the porous structure is prominent, indicating effective leaching at low salt concentrations (~10%). In contrast, the high-salt, non-BTO sample (Fig. 5(b)) shows a much denser distribution of pores, consistent with an increased NaCl content (~20%). The higher salt concentration led to more extensive pore formation and a more interconnected porous network. This suggests that the extent of porosity in the PDMS can be effectively tuned through salt concentration.
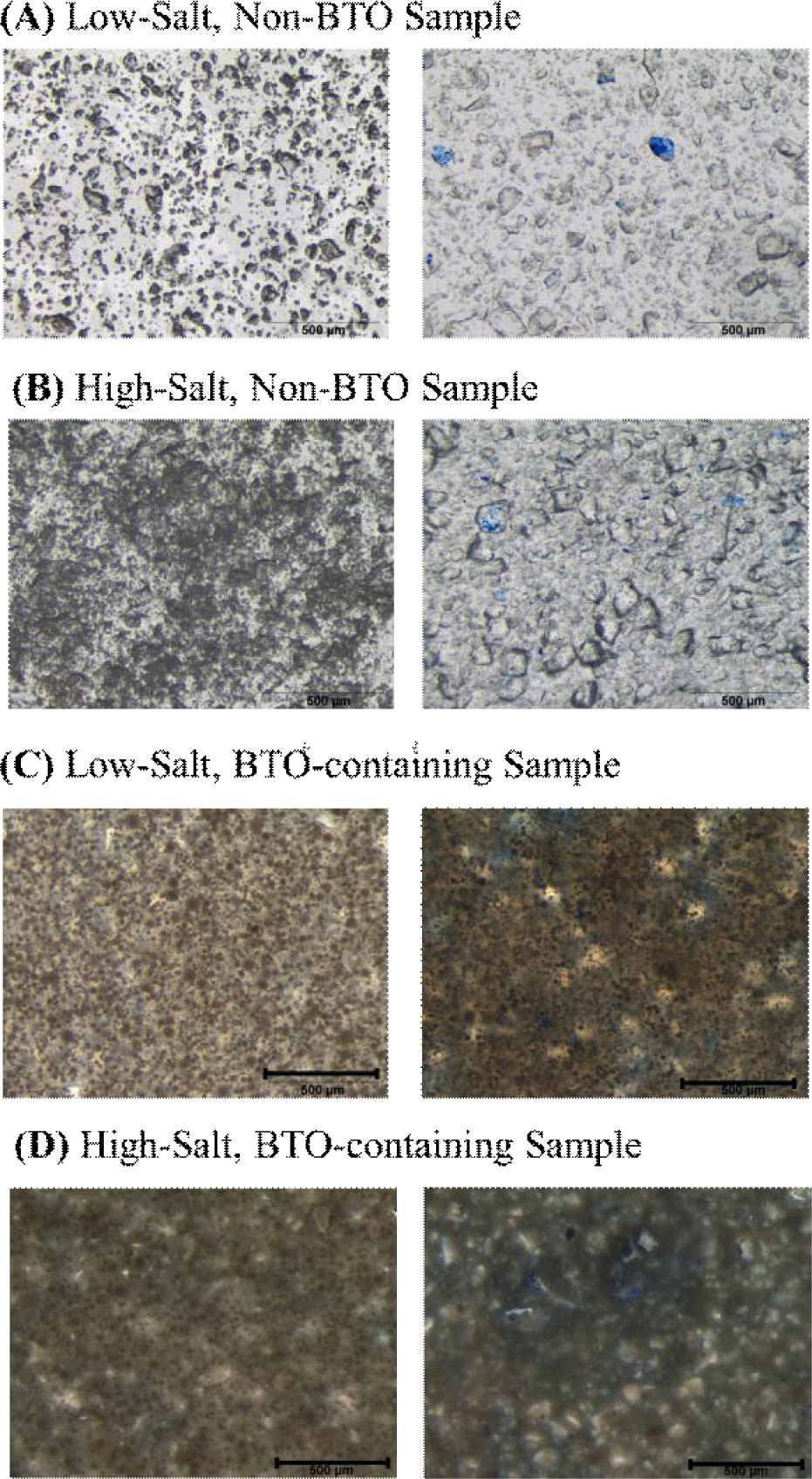
The incorporation of BTO nanoparticles notably alters the microstructure. In the low-salt, BTO-containing sample (Fig. 5(c)), the presence of BTO appears to reduce the clarity of individual pores compared to the non-BTO counterpart, possibly due to nanoparticle clustering around the salt domains. This behavior may stem from polar–polar interactions between BTO and NaCl, leading to partial aggregation or shielding effects. Despite this, the overall porous character remains evident. Finally, the high-salt, BTO-containing Sample (Fig. 5(d)) presents a densely packed microstructure where the porosity is less distinguishable by eye. The combination of high salt and BTO content results in a more uniform but optically darker matrix. BTO nanoparticles are likely concentrated near former NaCl sites, and such clustering could cause localized enhancement in dielectric properties. However, uniform distribution is limited, highlighting challenges in nanoparticle dispersion within highly porous systems.
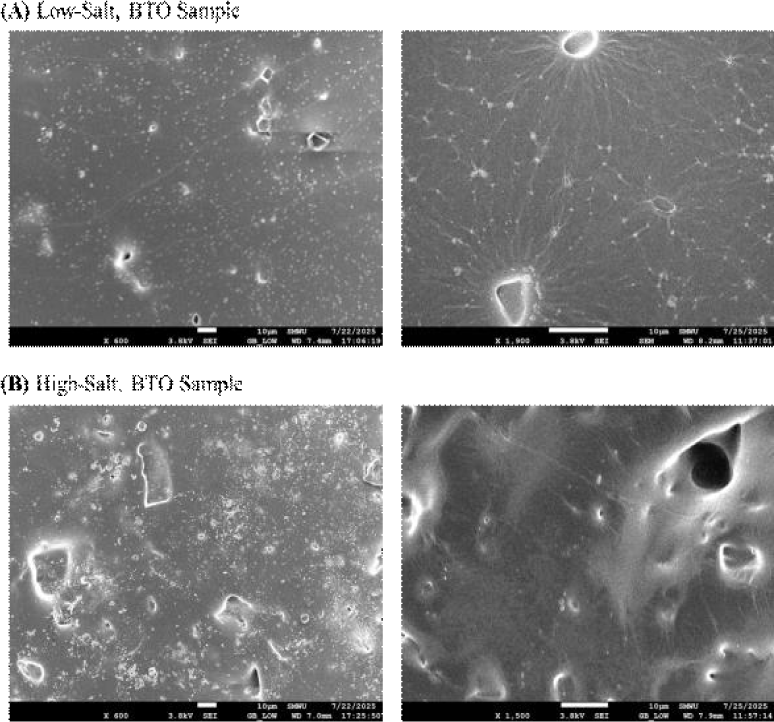
To examine the pore distribution further, we examined the samples using scanning electron microscopy (SEM). Fig. 6(a) shows the samples fabricated under low-salt conditions, while Fig. 6(b) shows composites with higher NaCl content. A clear increase in pore density is observed with higher salt loading, consistent with expectations based on sacrificial templating. This confirms that porosity within the PDMS matrix can be effectively modulated by adjusting the NaCl concentration. In addition, BTO nanoparticle agglomerates are visible as bright regions dispersed throughout the matrix. Despite the increase in pore density, the pore morphology appears highly heterogeneous, with irregular sizes and distributions. This non-uniformity highlights an area for further process refinement—such as improved salt sieving or mixing protocols—to achieve more consistent microstructures.
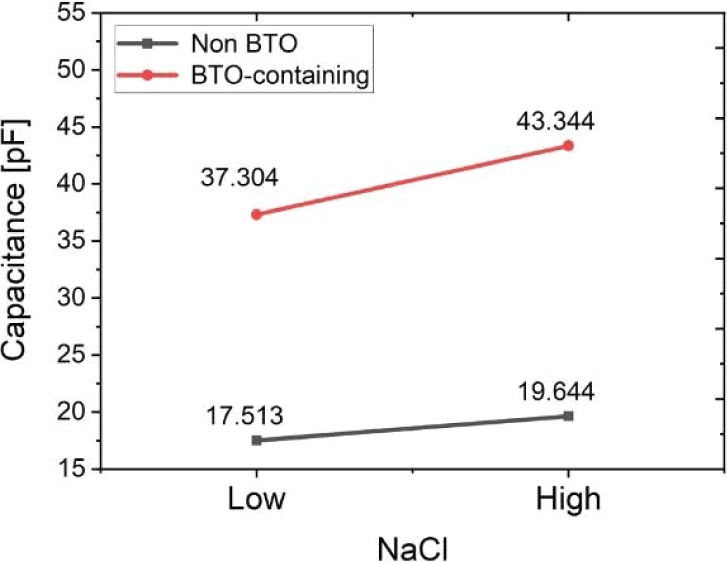
Sensors incorporating barium titanate (BTO) nanoparticles demonstrated significantly enhanced capacitance values compared to their non-BTO counterparts, validating the effect of dielectric enhancement. As shown in Fig. 7, under low NaCl-induced porosity conditions, the BTO-containing sensor exhibited a capacitance of approximately 37.3 pF, more than double that of the non-BTO sensor, which recorded only 17.5 pF. This trend persisted at higher porosity levels, where the BTO-containing sensor further increased to 43.3 pF, while the non-BTO sensor rose modestly to 19.6 pF. These results indicate that the presence of BTO substantially increases the dielectric constant of the composite, allowing for greater capacitance and improved sensitivity.to pressure-induced deformation. Interestingly, the higher NaCl concentration consistently led to higher capacitance values. While it is counterintuitive as the higher porosity would suggest more air within the dielectric, we tentatively assign this to the clustering of BTO near the pores induced by the ionic interactions.
Fig. 8 illustrates the pressure-responsive capacitive behavior of the porous BTO-integrated pressure sensor tested in the setup discussed in Fig. 4. In Fig. 8(a), the real-time measurements of pressure (P, black) and capacitance (C, red) are plotted over a duration of 120 seconds. A staircase pattern of increasing and decreasing pressure—from 0 to 14 mmHg and back—was applied to assess the sensor’s responsiveness. The capacitance closely follows the applied pressure steps, with clear and immediate changes in value corresponding to each increment and decrement in pressure. This synchronous behavior confirms that the sensor is capable of reliably converting physical pressure variations into measurable electrical outputs. The stability of the response across both loading and unloading cycles highlights the sensor’s repeatability and mechanical robustness.
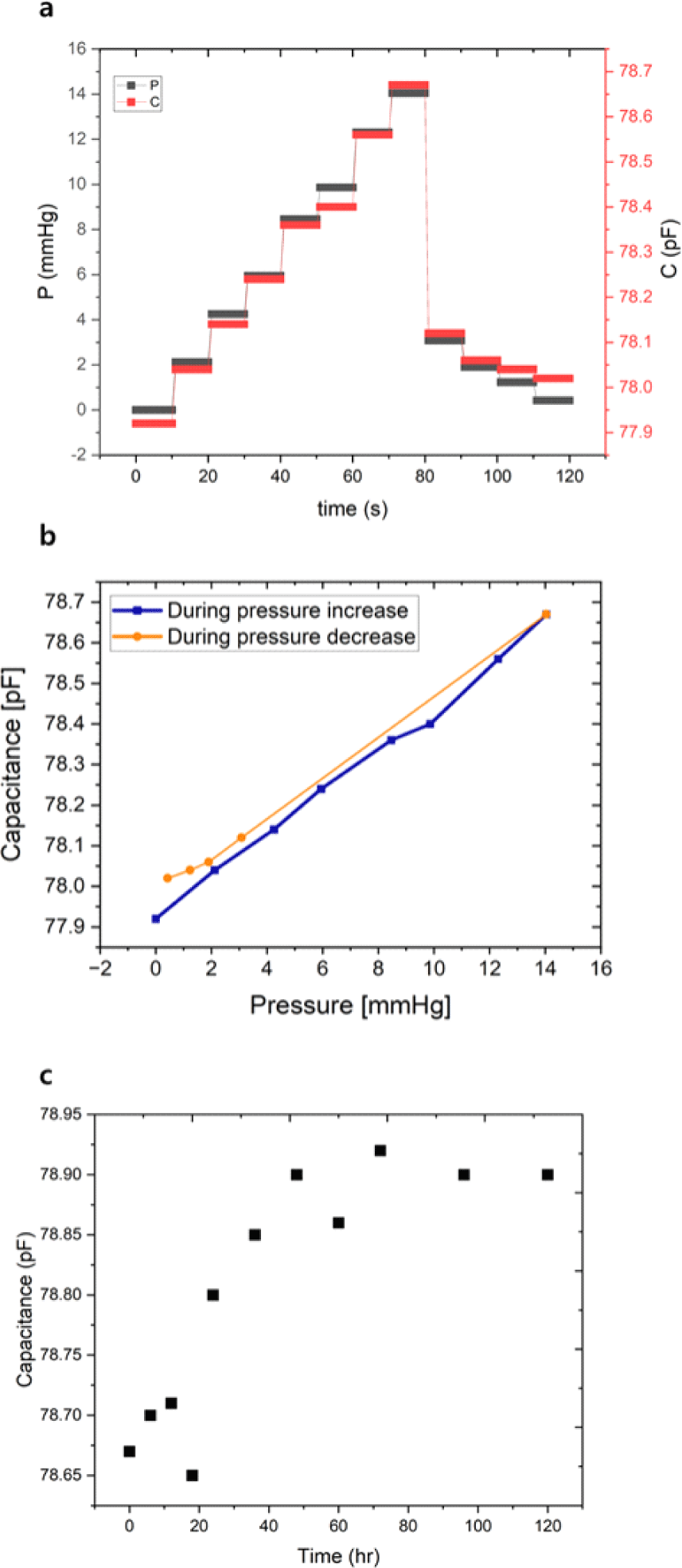
Fig. 8(b) further examines the relationship between capacitance and pressure by plotting capacitance values separately for the pressure-increasing and pressure-decreasing phases. A strong linear correlation is observed during the pressure increase, with a sensitivity of approximately 52 fF/mmHg and a coefficient of determination (R2) of 0.9943, indicating excellent linearity within the physiological pressure range. Although a minor hysteresis is noted during the unloading phase—where capacitance values are slightly higher than those during loading at equivalent pressures— this behavior is minimal and likely stems from viscoelastic relaxation of the porous PDMS matrix. Overall, the results demonstrate that the sensor possesses high sensitivity, strong linearity, and reliable performance under cyclic pressure conditions, making it well-suited for continuous intra-organ pressure monitoring applications. In addition, the sensor stability was examined. The capacitance changes as a function of time following the immersion in water is shown in Fig. 8(c), where the small capacitance drift is initially observed in the first 3 days but eventually stabilizes.
More notably, the sensor exhibits excellent linearity (R2=0.9943) across the 0–14 mmHg range, a feature that surpasses many reported soft sensors, which often show nonlinear behavior due to the viscoelastic properties of elastomers or electrode instability. The low hysteresis between loading and unloading cycles observed in this work is also an important improvement, as many porous PDMS-based systems exhibit pronounced mechanical memory effects. These attributes are particularly significant for intra-organ pressure monitoring applications—such as interstitial fluid pressure (IFP) in tumors, intracranial pressure, or intraocular pressure—where both sensitivity and consistency across repetitive measurements are crucial.
When compared to previously reported flexible capacitive pressure sensors [9-11], the performance of the proposed porous PDMS-based sensor integrated with BTO nanoparticles demonstrates a favorable balance of sensitivity, linearity, and reliability—particularly within the low-pressure range relevant to biomedical applications. The sensor achieves a sensitivity of approximately 0.0518 pF/mmHg (equivalent to~0.69 pF/kPa), which is within the range of reported values in literature, albeit slightly lower than that of some highly engineered microstructured designs. For instance, sensors employing micro-pyramidal or interdigitated architectures have demonstrated sensitivities exceeding 1.0 pF/kPa; however, these designs often target higher pressure ranges and involve complex fabrication steps that limit their integration into soft biological environments. In contrast, the current sensor maintains a simple fabrication strategy by leveraging NaCl-induced porosity and dielectric enhancement through BTO dispersion—enabling mechanical compliance while enhancing capacitive response.
Overall, while the absolute sensitivity may be modest compared to highly specialized designs, the proposed sensor’s combination of simplicity, high linearity, low hysteresis, and effective operation in the low-pressure regime addresses key challenges that are often unmet in more complex or rigid systems. This highlights its potential as a robust and practical platform for minimally invasive, continuous physiological pressure monitoring.
4. CONCLUSION
This study presents a porous BTO-PDMS-based flexible capacitive pressure sensor specifically engineered for continuous monitoring of hydrostatic pressure in biological environments. By leveraging a combination of dielectric enhancement from BaTiO3 nanoparticles and structural compliance introduced through NaCl-induced porosity, the sensor achieves high pressure sensitivity while maintaining mechanical flexibility. The sensor exhibits excellent linearity (R2= 0.9943), low hysteresis, and a practical pressure response within the clinically relevant range of 0–14 mmHg, making it suitable for applications such as intra-organ and tumor interstitial fluid pressure monitoring. Furthermore, the sensor’s integration into a cylindrical Guyton chamber supports its minimally invasive deployment and enhances signal fidelity by isolating ambient mechanical disturbances. Compared to conventional MEMS-based or microstructured polymer sensors, the proposed device offers a simpler fabrication process without compromising performance. These results position the sensor as a promising candidate for long-term physiological pressure monitoring, with future potential for integration into implantable diagnostic and therapeutic systems.